Physics
Contact Info
Mailing Address
Cleveland State University
Department of Physics
2121 Euclid Avenue, PHY
Cleveland, OH 44115-2214
Campus Location
Science Building (SI)
2399 Euclid Avenue, Rm. 112
Contact Us
Phone: 216.687.2425
Fax: 216.523.7268
physics.dept@csuohio.edu
REU Available Projects
Studying Volume Phase Transition of Polymeric Microgels
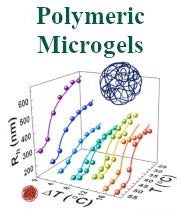
Dr. Kiril Streletzky: Polymeric microgels reversibly transition from large, soft particles of loosely bound polymer chains to smaller, tightly packed clusters at the volume phase transition. Their physical properties depend on environmental and synthesis conditions such as solution temperature, salt concentration, cross-linking density, and polymer properties. Careful synthesis allows to control the size distribution of microgels, which is beneficial for various applications. Microgels also provide a convenient testing ground for statistical mechanics models of gel thermodynamics such as the Flory-Rehner theory. A particular system of interest is the biocompatible polysaccharide, hydroxypropylcellulose (HPC). HPC-based microgels transition from a swollen state at room temperature to a de-swollen state above 41°C with a decrease in microgel volume by a factor of 3-20 depending on the synthesis protocol. However, heavily crosslinked microgels are found to unexpectedly grow with increase in temperature – a phenomena potentially attributed to non-uniform crosslinking of chains in the microgels. An REU student will study the effect of microgel synthesis conditions on microgel structure, dynamics, and density distribution by a combined dynamic and static light scattering at CSU and small angle x-ray scattering at Kent State University. The student will test observed microgel volume phase transition against modified Flory-Rehner theory. They will interact with Hore's group for phase transition modeling and with Fodor's lab for microgel wet imaging.
Confinement Effects in Polymer-Grafted Nanorod Solutions

Drs. Michael Hore and Kiril Streletzky: The conformation of polymers that are grafted to nanoparticles plays a significant role in determining the macroscopic physical properties of a material, such as mechanical and transport characteristics. Although the behavior of grafted polymers is well-understood for spherical cores (e.g., their conformation and relaxation dynamics), a comprehensive understanding of polymer-grafted, anisotropic nanoparticle shapes remains elusive to date. This knowledge gap is due to a lack of both measurements, analysis, and theory. Specifically, we do not definitively know how the different geometry of a nanorod surface impacts polymer conformation, and how a polymer grafted layer and/or free polymer chains in solution affect the translational and rotational diffusion behavior of anisotropic nanoparticles. An REU student will graft thiol-terminated polymers to Au nanorod surfaces through Au-S bonds and measure the rotational and translational diffusion coefficients with depolarized dynamic light scattering (DDLS). DDLS measurements will be performed for bare and grafted nanorods in water and polymer solutions in toluene/methanol that will index match the polymer so that only the nanorod dynamics are observed. The nanorod dimensions will be obtained from DDLS data using two home-made software packages (one by Hore and one by Streletzky). Refractive indices and refractive index increments (dn/dc) will be measured by differential refractometry. For this project a student will work in two labs: CWRU (nanorod synthesis, functionalization, refractometry) and CSU (DDLS, refractometry).
Design, Expression, and Characterization of Protein-based Materials
Dr. Nolan Holland: The Holland lab specializes in the design, synthesis, and characterization of functional protein-based materials. This work is ideal for introducing undergraduates to research due to various levels of complexity allowing projects to be designed at each student's level. Two project areas currently available to work on are 1) the design of nanoparticles for targeted drug delivery and 2) temperature responsive hydrogels for use as bio-inks in 3-D bioprinting. Specific student projects may include: a) using molecular biology to design and build genes that code for desired recombinant polypeptides, such as the incorporation of a new targeting motif onto nanoparticles or modifying the length of the polypeptides to change the thermal and/or mechanical properties of the resulting hydrogels; b) purification of the polypeptides and characterizing their assembly into nanoparticles or hydrogels using techniques such as light scattering in Streletzky's lab and SEM in Fodor's lab; c) characterization of physiochemical and/or mechanical properties of the nanoparticles and hydrogels using chromatography, optical spectroscopies, and rheometry.
Enhancing Electron Imaging Capabilities of Soft Matter Systems
Drs. Petru Fodor and Kiril Streletzky: Electron microscopy (EM) is now the "gold-standard" for high resolution morphological analysis of nano- and micro-scale systems. Unfortunately, using EM methodologies to characterize soft matter systems is difficult because: (i) the samples have to be conductive to dissipate the accumulation of charge during imaging; and (ii) the high vacuum and high energy electron beam within EM chamber are very harsh and can dry or thermally damage soft matter samples. Methods to mitigate these issues involve specialized sample preparation such as fixation, flash freezing, and staining that can distort the structure of the samples and reduce the resolution. Since these methods rely on permanent sample fixation, the dynamics of the systems is impossible to study. This is a big limitation for polymeric particles, where understanding their morphological response to environmental changes, such as temperature, is needed. To this end, we have pursued novel sample preparation methods to enable electron microscopy imaging of individual microgels, under conditions similar to their native environment, by either transferring samples into low vapor pressure liquids or sandwiching liquid samples between electron transparent membranes. In this project, students will: (i) design/fabricate imaging cells that preserve sample integrity while allowing EM visualization of nanoparticle dynamics; and (ii) develop image processing algorithms that remove the complex noise profiles associated with the beam and signal generation, and detection electronics. Students will become proficient in applying surface characterization tools for chemical/ morphological analysis and acquainted with computational image processing.
Phase Changes in Absinthe

Drs. Jessica Bickel and Andrew Resnick: Absinthe is typically drunk by adding cold water until it forms a cloudy "louche". This louche is a microemulsion formed by the competing interactions in this alcohol-water-oil system. Recent work has demonstrated that the turbidity of this louche is temperature dependent with a more turbid microemulsion in cold (15C) water and a less turbid microemulsion in warm (30C) water. Further, the dilution curve can be fit with a sigmoid shape and the fit parameters are linear with temperature. This continuing project will examine the effect of wormwood oil and anise flavoring in this microemulsion in two ways: (1) by pre-diluting with alcohol and measuring the louche and (2) by examining Bohemian absinthe, which has wormwood oil but not anise flavoring and does not form a louche. Students on this project will use: (i) optical transmission and scattering to characterize the turbidity of the louche in a temperature-controlled cuvette and (ii) rheometry and dynamic light scattering to determine the size of the particles in the microemulsion.
Synthesis of Organic Color Center-Tailored Carbon Nanotubes with Carbohydrate Functionality
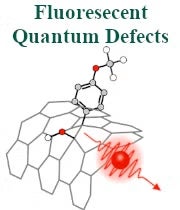
Dr. Geyou Ao: The recognition interactions between cell surface carbohydrates and carbohydrate-binding proteins (CBPs) play a vital role in a multitude of cellular activities, such as immune responses and infections. Understanding specific interactions between carbohydrates and CBPs has been challenging due to the lack of versatile probes. In this project, organic color centers-tailored single-wall carbon nanotube (OCC-SWCNTs) will be surface functionalized with carbohydrates to detect targeted carbohydrate-protein interactions. OCCs are fluorescent quantum defects that are implanted on the semiconducting SWCNT host. The intrinsic photoluminescence of OCC-SWCNTs in the near-infrared region will be used for optical detection of specific interactions with enhanced sensitivity and selectively. In this project, students will learn nanomaterial processing including dispersion, purification, surface functionalization and characterization techniques, such as optical spectroscopy.
Promoting Robust Crystallization of Organic Molecules via Row Surface Reconstructions

Dr. Jessica Bickel: Organic electronics are an interesting lower cost and eco-friendlier alternative to fragile inorganics that are typically used in electronic applications such as solar cells. But they suffer from lower conductivities. Partially or completely crystallizing these materials significantly improves conductivity while only slightly decreasing mechanical flexibility. In this project, we use row surface reconstructions to induce organic molecule crystallization. Surface reconstructions form as atoms rearrange at the surface to lower the surface energy and the resulting pattern repeats in a periodic fashion across the entire surface. The topography and bonding of the surface atoms defines a non-uniform but periodic energy landscape. This landscape determines how atoms diffuse on the surface and how they are incorporated into the surface. The goal of this project is to use row surface reconstructions to crystalize organic molecules without carefully pairing the molecules and surfaces. The students will use thermal evaporation to evaporate a variety of organic molecules from the acene and thiophene families onto these surfaces on order to examine how the molecules order. Further, these results will be compared to density functional theory (DFT) calculations performed by other REU or USRA students. DFT will be used to determine the thermodynamic minimum energy configuration and is accessible to undergraduate researchers who can begin calculations without fully understanding the code. Then they will examine the same molecules on the same surfaces being examined experimentally to determine whether ordering is driven primarily by topography or chemistry. Students can choose between an experimental or simulation project.
Crystallization of Anisotropic Particles

Drs. Jessica Bickel and Christopher Wirth: The ability to economically manufacture nano and micro-structure composites is critical, particularly if we can exploit the features of particles with shape and thus property anisotropy. Anisotropic materials have unique mechanical, electrical, and thermal properties intrinsic to individual particles. CNTs have been identified as a promising replacement for Silicon-based transistors in next-generation nanoelectronics; metal nanorods could be used in sensing applications because of the particles' interactions with light; and conductive polymers can be low-cost alternatives to expensive Si-based devices. However, current methods typically use magnetic or electric fields to assemble ellipsoidal particles, which will not work for all types of anisotropic particles. This work develops fundamental knowledge of crystallization in anisotropic materials using blade-coating. We have previously used a blade-coater to get crystalline regions of both 1mm and 0.5mm polystyrene (PS) beads. With a blade-coater, we can examine the crystallization as a function of both the aspect ratio of the particles and the deposition parameters, such as the blade speed, and angle. Ultimately, being able to consistently crystallize particles other than mono-disperse spheres will allow us to build more interesting devices. The students will crystallize PS beads stretched into ellipsoids with different aspect ratios and then examine the effect of PS ellipsoid surface chemistry on crystallization and bi-modal distributions of particles. Samples will be made at CWRU with Wirth and characterized at CSU with Bickel using Fodor's SEM.
Mathematical Modeling and Simulation of Active Biosystems and Biomaterials
Dr. Shawn Ryan: Self-organization is a fascinating process manifested in biological systems that results in interesting physical phenomena such as enhanced movement/mixing, efficient foraging, and striking effective properties in materials. While these striking features can be readily observed, there remains a critical need to identify the main microscopic interactions leading to its emergence. We seek to meet this need by using mathematical modeling, analysis, and simulations studying collective swimming of microscale organisms. This work will focus on the interactions at the microscale that lead to large-scale pattern formation as well as to novel effective material properties. This understanding will then be applied to the emerging field of biomaterials to study how materials such as biofluids exhibit enhanced functionality due to the presence of a self-organizing. The principal outcome is a fundamental understanding applicable to a wide range of similar organisms, many of which are anisotropic. These organisms include sperm, Janus particles, colloidal membranes, and granular matter, which are all capable of self-organization. One can even go to the nanoscale with chromosome dynamics within cells. This approach can also be used to develop microfluidic devices, drug-delivery systems, and biomaterial networks. Any models developed will be guided by experiments in potential collaboration with Dr. Wirth's lab or Dr. Kothapalli's Lab.
Microfluidic Reactors and Emulsifiers

Drs. Chandra Kothapalli and Petru Fodor: Our labs examine the design and implementation of microfluidic platforms to investigate experimentally and computationally complex physical (e.g., fluid mixing, chemical reactions) and biological (e.g., cell-cell, cell-matrix interactions, DNA folding) phenomena. Microfluidic devices offer great versatility with varying channel dimensions and architectures, flexibility to gradually introduce compounds and cells of interest, reduced consumption of samples and reagents, provision for in situ imaging and live-monitoring of experiments over long durations, enhanced reaction speed due to increased surface to volume ratios, high-throughput parallel sample processing, and high-content imaging capabilities. Moreover, they allow the implementation of unique methodologies from droplet microfluidics for chemical and particulate synthesis and analysis, to paper-based analytical devices for point of need detection. The student(s) involved will be engaged in the optimization and development of various microfluid platforms and/or the comparison of their functionality with corresponding batch reaction systems. They will be trained on the development and use of microfluidic platforms via: analytical and/or numerical modeling and optimization of the reactant flow and mixing, prototype fabrication using advanced manufacturing tools such as soft-lithography, functionality testing of the quality of mixing using optical imaging, and product assessment using chemical and electron microscopy analysis.
Flowing Past Cilium

Dr. Andrew Resnick: This laboratory focuses on cellular mechanosensation, specifically the sensing of extracellular fluid flow by ciliated epithelial monolayers. Students will fabricate a novel tissue culture perfusion chamber using "soft lithography", apply pre-defined steady and oscillatory fluid flow to ciliated cells cultured within the channel, and image the internal flow field and cellular response (intracellular Calcium and/or nitric oxide) with fluorescence microscopy. They will also stimulate primary cilia directly using optical trapping, applying localized forces directly to individual cilia and imaging the cellular response (intracellular Calcium and/or nitric oxide) with fluorescence microscopy. The mechanical stiffness of cilium will be chemically manipulated, with cilium stimulated either with fluid flow or with optical trapping, and the effect on sensor sensitivity will be assessed by live-cell Calcium imaging. Students will learn basic fluid mechanics (Reynolds & Strouhal numbers), cell physiology (ciliary mechano- sensation & directed salt/water transport), biophysical laboratory techniques, microscopy, quantification of a flow velocity field, and fluid-structure interaction. Knowledge of the internal flow-field within cell-free microchannels will be used in collaboration with Fodor to determine mixing rates and feedback for analytical and computational modeling. This experiment will yield a mechanistic understanding of cellular mechano-sensation, inspiring new microfluidic applications and will be supplemented by modeling with COMSOL.
Waste to Energy via Chemical Reactions in Multiphase Environments

Dr. Jorge Gatica: The ever-increasing problem of waste management calls for alternatives to landfill or incineration. Waste gasification, which breaks down long-chain-carbon-based materials into synthetic gas, is an attractive alternative for sustainable living environments. Catalytic processes investigated at CSU have shown promise as an efficient route to gasify solid residuals.
Wet-Thermal Catalytic Oxidation (WTCO) is a lower temperature (300-350 oC) and energy-demanding option to incineration (T>1000 oC). Unfortunately, some substrates found in waste, remain solid at low temperatures. This creates a heterogeneous reacting environment where catalyst particles, substrates, and oxygen interact. The fluid dynamics of a stratified reacting system in a high pressure stirred reactor presents a formidable problem. In this project, experiments are combined with computational fluid dynamics (CFD) for characterization and optimization. The gasification of long-chain polymers typically found in municipal and space exploration waste in a low-to-mid temperature/high pressure Stirred-Tank Reactor (STR) is studied. This unit does not allow flow visualization, therefore, CFD modeling is essential for process optimization. The STR has tangential liquid motion that leads to the formation of a central vortex on the liquid free surface. These forces may control particle dynamics and lead to stratification.
The goals are to:
- a) model the particle dynamics in WTCO of waste using a finite-element method;
- b) use in-silico experiments to characterize chemical reaction and transport phenomena in particulate systems;
- c) characterize catalytic gasification dynamics for different substrate and catalyst particulates;
- d) scale up to continuous gasification.
The student will learn the fundamentals of fluid dynamics of slurry systems, the interrelation between fluid dynamics and transport phenomena in reacting media for gasification modeling, and low-to-mid temperature and high-pressure reactor operation with in-silico experiments for gasification optimization.


Contact Info
Mailing Address
Cleveland State University
Department of Physics
2121 Euclid Avenue, PHY
Cleveland, OH 44115-2214
Campus Location
Science Building (SI)
2399 Euclid Avenue, Rm. 112
Contact Us
Phone: 216.687.2425
Fax: 216.523.7268
physics.dept@csuohio.edu