Chemistry
Contact Info
Mailing Address
Cleveland State University
Dept. of Chemistry
2121 Euclid Ave., CHM
Cleveland, OH 44115-2214
Campus Location
Science & Research Center (SR)
2351 Euclid Avenue, Rm. 397
Contact Us
Phone: 216.687.2451
Fax: 216.687.9298
m.r.jones@csuohio.edu
Description of the ComACC-accredited doctoral program in Clinical
Overview
The doctoral program in Clinical Chemistry is a specialized track in the Clinical-Bioanalytical Chemistry Ph.D. degree program, which is a joint degree program of Cleveland State University (CSU) and the Cleveland Clinic. The doctoral degree program was approved by the Ohio Board of Regents in 1998 as a restructured program between the Department of Chemistry at CSU and the Lerner Research Institute at the Cleveland Clinic. Prior to that, the doctoral program in Clinical Chemistry was approved by the Ohio Board of Regents in 1973. The doctoral program track in Clinical Chemistry is presently accredited by the Commission on Accreditation in Clinical Chemistry (ComACC) and has been for most of its 50 years. CSU’s program is presently the only accredited doctoral program in Clinical Chemistry, both nationally and internationally.
The doctoral program in Clinical Chemistry is a dynamically integrated program merging the fields of biomedicine, clinical diagnosis and analytical chemistry. The instructional and training components are carried out by clinical chemistry, analytical chemistry and pharmaceutical science faculty in the Department of Chemistry at Cleveland State, with active involvement of clinical chemists and clinical scientists at the Cleveland Clinic, University Hospitals Case Medical Center, the MetroHealth System, Akron Children’s Hospital and other area medical institutions.
Mission and Objectives of the Program
The mission of the doctoral program in Clinical Chemistry is to give Ph.D. graduate students intensive didactic instruction in the field of clinical laboratory science and to give them appropriate biomedical research experience in their dissertation work, in order to prepare students for one of the following paths upon graduation:
- To obtain further practical training in the field through clinical chemistry post-doctoral fellowships, in order to prepare them for careers as directors of clinical laboratories;
- To directly assume other scientific positions in clinical laboratories, either at medical institutions or reference laboratories;
- To assume positions in the in-vitro diagnostics, pharmaceutical or biotechnology industries, which are increasingly seeking scientists with knowledge of clinical chemistry
The program sets high standards of excellence in delivering its curriculum to prepare students for these careers. Knowledge of both clinical aspects and interpretation of test results, as well knowledge of analytical techniques and various aspects of quality operation in the clinical laboratory, are central to the program’s goals and mission. Additionally, the program mentors students to become independent researchers, educating and mentoring the student in dissertation research projects involving cutting-edge analytical methodologies and novel clinical/biomedical research, under the direction of faculty from Cleveland State and the Cleveland Clinic. A particular analytical strength of the program is the dissertation research utilizing core mass spectrometry instrumentation at CSU and the Cleveland Clinic. Finally, the program involves students in the activities of the NEOhio American Association for Clinical Chemistry, fostering their professional development. Graduates from this track receive a Graduate Certificate in Clinical Chemistry upon meeting standards of performance in the curriculum.
Accomplishments of the Program
Overview of Graduates and Their Positions
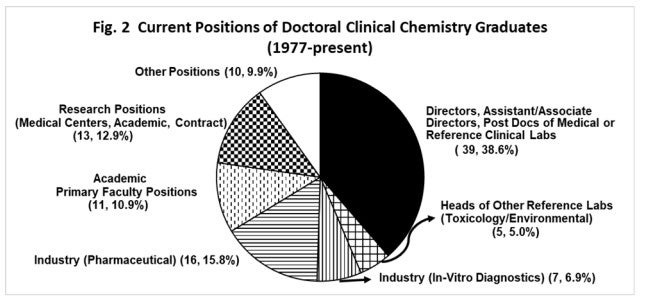
The doctoral program in clinical chemistry program prepares doctoral students for careers as directors of clinical laboratories, directors of other laboratories, scientists in industry, research scientists and primary academic faculty positions. Since its inception, there have been 101 graduates from the doctoral program in clinical chemistry. The types of positions currently held by our graduates include: directors/co-directors/post-doctoral fellows of clinical laboratories (both in medical centers and reference medical laboratories; 39%), heads of other reference laboratories (toxicology/environmental; 5%), scientists in the in-vitro diagnostics (7%), and pharmaceutical industry (16%), faculty at academic institutions (primary position) (11%), research scientist positions (13%), and other positions (10%). High profile positions included (some have retired): three CEOs (San Diego Reference Laboratory, Medical Specialties, Cardiovascular Clinical Sciences); three vice-presidents (Senior Vice President at Quest Diagnostics, at Quest-Cleveland Heart Lab and at Siemens Healthineers); 19 heads of clinical labs at medical facilities, including Mayo Clinic, Yale, University of Massachusetts, Vanderbilt, Houston Methodist Hospital, Tulane, Cleveland MetroHealth Medical Center and Cleveland Veterans Administration Medical Center; and 11 primary appointment faculty positions, including positions at University of Chicago (formerly Harvard), Old Dominion University, Boston University School of Medicine, Cleveland State, and a dean at Malone College. Two CSU graduates have been elected in 2021 to AACC national positions; Joe El-Khoury to the Board of Directors and Linnea Baudhuin to the Nominating Committee. Three CSU graduates are co/associate directors of ComACC clinical chemistry post-doctoral programs (Joe El-Khoury at Yale, Xin Yi at Houston Methodist Hospital and Joe R. Wiencek at Vanderbilt).
Lists of recent Ph.D. graduates completing the accredited program who secure clinical chemistry post-doctoral training fellowships. Eighteen graduates from the doctoral clinical chemistry program secured highly competitive post-doctoral fellowships in clinical chemistry in the period 2012-2021.
Recent Ph.D. Graduates from CSU-CC Doctoral Program In/Completed ComACC Clinical Chemistry Post-Doctoral Programs
Graduation Year & Present position
Graduate (2023)
University of Chicago Medicine
Graduate (2023)
University of California, San Francisco
Graduate (2021)
University of Washington
[Associate Director of Clinical Chemistry, University of Rochester Medical Center]
Graduate (2021)
University of Utah-ARUP
Graduate (2019)
Yale University/Yale New Haven Health (July 2021)
[Director of Special Chemistry and Toxicology, University of Kentucky College of Medicine]
Graduate (2019)
Cleveland Clinic (March 2020)
[Assistant Director, Clinical Chemistry, Duke University Health System Clinical Laboratories]
Graduate (2019)
University of Louisville (July 2019)
[Clinical Laboratory Director, Tulane University School of Medicine, New Orleans, LA]
Graduate (2019)
Mayo Clinic (July 2019)
[Technical Director of Chemistry, Toxicology, and Point of Care, University of Cincinnati Health Center]
Graduate (2019)
Yale University/Yale New Haven Health (July 2019)
[Technical Laboratory Director, Sanford Laboratories/ Sanford Health, Sioux Falls, SD]
Graduate (2016)
University of Minnesota (July 2018)
[Clinical Chemist, Primary Children’s Hospital and Intermountain Central Laboratory, Murray, UT]
Graduate (2017)
Mayo Clinic (July 2017)
[Director of Clinical Chemistry and Immunology, Department of Pathology, Spectrum Health, Grand Rapids, MI]
Graduate (2016)
Medical University of South Carolina (July 2017)
[Technical Director, Toxicology, Pathology Department, Beaumont Health, Beaumont, MI]
Graduate (2016)
Yale University/Yale New Haven Health (July 2017)
[Assistant Director, Clinical Chemistry and Laboratory Informatics, Nationwide Children's Hospital, Columbus, OH]
Graduate (2016)
Baylor College of Medicine and Texas Childrens Hospital (July 2016)
[Section Head – Clinical Chemistry, Toxicology, MetroHealth Medical Center, Cleveland, OH]
Graduate (2016)
University of Utah – ARUP (July 2016)
[Director of Analytical Operations, Doctor’s Data, Inc (Specialty Testing Clinical Lab) St. Charles, IL]
Awards and Other Accomplishments by students
In a recent six-year period, students have garnered thirty-seven national meeting awards/recognitions and ten significant local scientific and institution awards given to 22 different students. There are a total of 16 awards for research at the national level. This includes 13 research, abstract, and oral presentation awards at national AACC meetings that includes AACC Academy Distinguished Abstract Awards (4), Best Abstract awards from AACC divisions (4), the selection of two students to make oral presentations at the Hot Topics in Clinical Chemistry session, and Student Research Awards (3), including one first-place finish at the Oral Abstract Competition. Other national research awards includes two research awards at other scientific meetings and national recognition of an outstanding publication. Local and institutional research awards include three at Cleveland State at the university level, one Cleveland Clinic Best Poster award and one outstanding speaker award at the Cleveland section of the American Chemical Society meeting. The other awards are travel and young investigator awards to travel to national AACC, MSACL and other scientific meetings and several Cellular and Molecular Medicine Pre-doctoral Fellowship Awards at Cleveland State.
In the past five years, there have been 28 poster presentations given by 16 different doctoral students at national AACC and MSACL meetings.
Description of Curriculum
The required courses in the doctoral clinical chemistry program are given in Table 2, with catalog summary of topics covered given in Table 3. The other requirements for all chemistry Ph.D. students are related to dissertation research passing a candidacy exam, presenting annual research reports to their dissertation committee members, conducting dissertation research and writing and successfully defending their dissertation. In all, a total of 90 credit hours of course is required for the Ph.D.
Requirements of the Ph.D. Program in Clinical Chemistry
| Course | Semsester | Credits per course |
|---|---|---|
| Clinical Chemistry I (CHM 651) | Fall, Year 1 | 4 |
| Clinical Chemistry II (CHM 652) | Spring, Year 1 | 4 |
| Advanced Biochemistry I (CHM 653) | Fall, Year 1 | 4 |
| Advanced Biochemistry II (CHM 654) | Spring, Year 1 | 4 |
| Special Topics in Clinical Chemistry (CHM 750) | Spring Year 2 and after | 1, 4 courses |
| Clinical Laboratory Topics: Instrumentation and Quality Operation (CHM 658/758) | Spring Year 2 | 4 |
| Internship in Clinical Chemistry (CHM 756) | Year 3 or after | 3 |
| (Choose one course below) Biotechnology Techniques (CHM 655) Pharmaceutical Analysis Laboratory (CHM 557) | Fall, Year 2 | (CHM 655) 4 (CHM 557) 3 |
| Chemistry Seminar (CHM 695/795) | Any semester | 1, 2 courses |
| Clinical Chemistry Seminar (CHM 759) | Any semester | 1, 2 courses |
| Candidacy Exam (CHM 891) | Fall Year 3 | 1 |
| Annual Research Report (CHM 790) | Every year after CHM 891 | 1 each year |
| Advanced Chemistry Laboratory (CHM 779) | Dissertation research | 89 – above total credits |
| Ph.D. Dissertation (CHM 899) | Dissertation defense | 1 |
Total | 90 |
Summary Descriptions of, and Recommended Schedule, for Taking
Courses in the Doctoral Program in Clinical Chemistry
CHM 651 Clinical Chemistry I (4 credits). Laboratory diagnosis of kidney, liver, hemolytic anemias, electrolyte, acid-base disorders. Instruction includes physiology and pathophysiology in conjunction with laboratory testing for the above diseases. (Fall Year 1)
CHM 652 Clinical Chemistry II (4 credits). Clinical enzymology and enzyme assays, body fluids, bone (PTH, calcium, vitamin D) disorders, heart disorders, cardiovascular risk, pancreatic and GI disorders, lipoproteins and lipids, immune disorders, arterial blood gases, endocrine disorders, immune disorders, infectious diseases, and molecular diagnostics. Instruction includes physiology and pathophysiology in conjunction with laboratory testing for the above diseases. (Spring Year 1)
CHM 653 Advanced Biochemistry I (4 credits). Structure and function of proteins, metabolism of carbohydrates and lipids, bioenergetics and enzymes. (Fall Year 1)
CHM 654 Advanced Biochemistry II (4 credits). Degradation of nitrogen-containing compounds, signaling, DNA and RNA processes, protein synthesis and degradation, molecular biology techniques, cancer, genetic diseases. (Spring Year 1)
CHM 750 Special Topics in Clinical Chemistry (1 credit). One of four topic areas in clinical laboratory covered each time course is offered. The entire four-part series covers topics of tumor markers, hematology, molecular diagnostics, therapeutic drug monitoring, pharmacology, toxicology, pregnancy, prenatal diagnosis, inborn errors of metabolism, clinical nutrition, thyroid disorders, and laboratory management. (Spring Year 2 and after, 4 courses required)
CHM 658 Clinical Laboratory Topics: Instrumentation and Quality Operation Topics (4 credits) Course covers quality control and quality assessment issues of the clinical laboratory, statistical methods in laboratory medicine, reference intervals, evidence-based laboratory medicine, sample preparation, analysis of different sample types (blood, urine, CSF, amniotic) pre-analytical variability, post-analytical phase, proficiency testing, regulatory issues, as well as covering types of analytical instrument technology that are used in the clinical laboratory, including; spectrophotometry, fluorometry, immunoassays, electrophoresis, mass spectrometry, refractometry, osmometry, electrochemical and proteomic techniques. (Spring Year 2)
CHM 756 Internship in Clinical Chemistry I (3 credits). Rotation through the clinical laboratory at a Cleveland/Akron medical centers. Areas covered include principles of instrumentation, chemistry of analysis, the clinical uses of analytes, quality control, calibration, maintenance, sample preparation and management issues in the laboratory. The internship may also include doing a clinical laboratory project. (Year 3 or after)
CHM 655 Biotechnology Techniques (4 credits). Techniques of immunoassays, and techniques of isolation, manipulation, and analysis of proteins/nucleic acids are covered. Includes both lecture and laboratory. (Fall Year 2) (either this course or CHM 557)
CHM 557 Pharmaceutical Analysis Laboratory (3 credits) Hands-on experience for the major instrumentation and techniques used in the analysis of pharmaceuticals and biopharmaceuticals. The laboratory experiments involve the analysis of drug molecules by atomic emission spectroscopy, fluorometry, infrared spectroscopy, nuclear magnetic resonance spectroscopy, gas chromatography, liquid chromatography, and mass spectrometry, as well as methods of sample preparation. (Fall Year 2) (either this course or CHM 655/755)
CHM 759 Clinical Chemistry Seminar (1 credit). Seminars on a range of clinical laboratory topics, diagnosis of disease, issues in the clinical laboratory. (Taken any semester, 2 courses required)
Faculty
Cleveland State University – Department of Chemistry
David Anderson, PhD, DABCC
HPLC and mass spectrometry: proteins, pharmacokinetic studies, gangliosides,
HPLC development and innovation, chromatographic theory
Mekki Bayachou, PhD
Functional biomaterials; antithrombotic surfaces; electron-transfer; nitric oxide synthases; metalloproteins; metalloenzymes; cyt P450s; bio-electrochemistry; DNA-sensors; DNA-protein interaction; small molecule metabolite sensors; nanotechnology
Anthony Berdis, PhD
DNA replication; chemotherapy; mutagenesis; cancer biology; nucleoside analogs; pharmacology; drug discovery; medicinal chemistry
Warren Christopher Boyd, PhD
Transition metals; coordination chemistry - manganese, cobalt, nickel, copper, zinc, aluminum, nitrosoarenes, azodioxides; spectroscopy; electrochemistry; catalysis; redox-active ligands; apoptosis; anticancer agents
Valentin Gogonea, PhD
Computational chemistry; enzyme reactivity; nitric oxide synthase; hydrogenase; cholesterol transport; deuterium exchange; quantum mechanical molecular mechanics; molecular dynamics; protein folding
Michael Kalafatis, PhD
Thrombosis; haemostasis; coagulation factors; factor V; factor X; prothrombin; prothrombinase; thrombin; platelets; endothelial cells; kinase; phosphorylation; signal transduction; cancer; cell division; apoptosis; kinase; lupus anticoagulant
Yana Sandlers, PhD, DABCC
Clinical chemistry; mass spectrometry; inborn errors of metabolism; metabolomics; induced stem cells derived cardiomyocytes (iPSCM); Barth syndrome; cardiomyopathy
Bin Su, PhD
Drug development; cancer; African trypanosomiasis; synthetic medicinal chemistry; pharmacokinetic studies
Xue-Long Sun, PhD
Pharmaceutical chemistry; chemical biology; biopharmaceutical chemistry; cellular chemistry; glyco-engineering; antithrombtic and antiviral drugs; immunomodulation
John Turner, II, PhD
Raman spectroscopy; fluorescence; infrared; IR; near infrared; NIR; visible; chemical imaging; multivariate analysis; chemometrics; mineral; biomineral; bone; teeth; biomaterials; implant materials; bioanalysis; spectral imaging; hyperspectral imaging; multispectral imaging; liquid crystal tunable filter; LCTF; acousto-optic tunable filter; AOTF; CCD; charge coupled device, spectroscopy; gemology; gemstone; mineralogy; geology; Raman database; poly-l-lactide
Aimin Zhou, PhD
Investigating the role of RNase L, a key enzyme in interferon action against viral infection and cell proliferation, in immune cell functions and the pathogenesis of inflammatory diseases such as diabetes, non-alcohol fatty liver disease, and acute kidney injury by using cell and animal models.
Cleveland Clinic—Robert J. Tomsich Pathology and Laboratory Medicine Institute
Edmund Z. Reineks, MD/PhD, DABCC
Point of care testing; quality; lab directorship; special topics
Jessica Colon-Franco, PhD, DABCC
Endocrinology; special chemistry; tumor markers; inflammatory biomarkers; leadership
Kamran Kadkhoda, PhD, DABMLI
Immunopathology; serology; allergy; cellular immunology
Grace Kroner, PhD, DABCC
Special chemistry; diabetes testing; laboratory test utilization; data analytics
Adam McShane, PhD, DABCC
Automated chemistry; cardiovascular disease; blood gases
Marvin Natowicz, MD/PhD
Biochemical genetics; metabolic diseases
Cleveland HeartLab (Quest Diagnostics)
Deborah Sun, PhD
Clinical laboratory operations and management
MetroHealth Medical Center
Mahesheema Ali
Clinical chemistry, toxicology, POCT, rapid response labs
Cleveland Clinic – Lerner Research Institute/Other
Ashok Agarwal, PhD (American Center for Reproductive Medicine)
Biological markers of oxidative stress; proteomic and bioinformatics in DNA damage, apoptosis and preserving fertility in patients with cancer; laboratory and clinical studies assessing efficacy of certain antioxidants in improving male fertility
Suneel Apte M.B.B.S ,PhD (Biomedical Engineering)
Extracellular matrix and matrix-degrading proteases in osteoarthritis and inflammatory arthritis, heart disease, aortic aneurysms; genetic disorders affecting heart, eyes, and limbs; biochemistry, cell biology, genetic and proteomic technologies
Mark Aronica MD (Inflammation & Immunity)
Components of the extracellular matrix in development and pathogenesis of asthma.
Kathleen Berkner, Ph.D. (Cardiovascular and Metabolic Sciences)
gamma-glutamyl carboxylation of vitamin K-dependent proteins in hemostasis, calcification, apoptosis, signal transduction and growth control; mouse models; proteomics; mutagenesis; cellular systems; biochemical studies
Mark Brown, PhD (Cardiovascular and Metabolic Sciences)
Diet-microbe-host interactions in obesity, diabetes, cardiovascular disease, and cancer; diet and gene interactions non-alcoholic fatty liver disease (NAFLD) and alcohol-associated liver disease; nutrition and obesity mechanisms in gastrointestinal cancers.
Tatiana Byzova, PhD (Neurosciences)
Neoangiogenesis; angiogenesis in wounds, cancer, ischemia; vascular dysfunction in aging and neurodegenerative diseases; cancer and inflammation-associated vascular pathologies and thrombosis; ex vivo and in vitro cell and molecular biology; advanced imaging; analysis of model and clinical samples
Jan Claesen, PhD (Cardiovascular & Metabolic Sciences)
Defining signaling systems used as intra-and inter-bacterial communication in the gut and skin, and understanding the interactions between dietary components and the microbiota in the context of disease prevention. Identifying therapeutic targets that modulate the microbe–microbe and microbe–host cell interactions; microbiology; bacterial genetics; synthetic biology; small molecule biosynthesis; biochemistry
Donna Driscoll, PhD (Cardiovascular & Metabolic Sciences)
Selenoproteins: anti-oxidant defense, thyroid hormone metabolism, development, muscular and nervous system function, inflammation, and cancer. Selenoprotein synthesis and pathway effects of selenium deficiency.
Serpil Erzurum, MD (Inflammation & Immunity)
Mechanisms of lung inflammation and remodeling that lead to lung disease; mechanisms of abnormalities in redox balance, signal transduction, accelerate arginine metabolism and impact nitric oxide (NO) production
Paul Fox, PhD (Cardiovascular and Metabolic Sciences)
IFN-gamma signaling; mechanisms of blood vessel formation; ferroxidases in iron metabolism in inflammatory diseases and chronic renal failure
Stanley Hazen, MD, PhD (Cardiovascular and Metabolic Sciences)
Inflammation in cardiovascular diseases; myeloperoxidase in oxidant stress and cardiovascular diseases; HDL structure and function; intestinal microbiota in cardiometabolic disease; bench-to-bedside; basic/genetic, cellular, animal model, and human clinical investigations; variety of analytical methods including mass spectrometry
Christopher Hine, PhD (Cardiovascular and Metabolic Sciences)
Hormonal and nutritional regulation of endogenous hydrogen sulfide (H2S) production and metabolism; H2S effects in biological pathways; regulation and function of H2S generating enzymes; diet, exercise, and pharmaceuticals harnessing endogenous H2S production for increased stress resistance, metabolic fitness, and lifespan.
Sadashiva Karnik, PhD (Cardiovascular and Metabolic Sciences)
Angiotensin II type 1 receptor (AT1R) structure, function, physiology, genetics and signaling; hypertension; cardiac hypertrophy; heart failure (HF); Approaches include transgenesis, molecular pharmacology, ligand design, membrane protein biochemistry, protein-protein interaction, signal transduction, gene regulation, micoRNA regulation, proteomics and posttranslational modifications
Xiaoxia Li, PhD (Inflammation and Immunity)
Signal transduction in innate and adaptive immunity, IL-1 receptor/Toll-like receptor (IL-1R-TLR) signaling mechanisms, Act1 regulating autoimmunity through T- and B-cell-mediated immune responses
Daniel Lindner, MD, PhD (Translational Hematology and Oncology Research)
Mechanisms of acquired drug resistance in renal cancer, drugs for myelodysplastic syndrome and acute myelogenous leukemia
Thomas McIntyre, PhD (Cardiovascular and Metabolic Sciences)
Lipid mediators in the innate immune or inflammatory system; phospholipid PAF activation of inflammatory cells in cardiovascular and renal systems; oxidized phospholipids in apoptosis, affecting platelets in thrombosis and vascular disease; acute kidney disease
Ina Nemet, Ph.D
Gut microbial metabolism and relationship between microbial metabolites and disease risks; conventionally raised and/or germ free host colonized with genetically engineered human commensals to demonstrate direct effects of particular enzyme/metabolite on the host; metabolomics (mass spectrometry) and clinical studies in tandem with mechanistic studies
Richard Padgett, PhD (Cardiovascular and Metabolic Sciences)
Mechanisms of post-transcriptional RNA processing, pre-mRNA splicing; myelodysplastic syndrome or acute myeloid leukemia; mutations in a small RNA (RNU4ATAC) resulting in growth retardation, microcephaly and skeletal deformities; genetic signals promoting correct splicing of giant introns
Eugene Podrez, MD, PhD (Cardiovascular and Metabolic Sciences)
Inflammation and oxidative stress in atherosclerosis and thrombosis via lipid peroxidation and formation of bioactive lipids; induced platelet hyperreactivity associated with dyslipoproteinemia; platelet scavenger receptors type B targeted for new therapies; mechanisms of lipid oxidation products impairing cholesterol efflux inducing intracellular cholesterol accumulation
Jun Qin, PhD (Cardiovascular and Metabolic Sciences)
Protein-protein interactions in heart failure, diabetes and cancer; integrins in cell adhesion, morphology, and motility; mechanisms of integrin-mediated protein interaction in signaling; structural biology techniques of NMR and crystallography
Sujata Rao, PhD (Ophthalmic Research)
Circadian regulation of neuronal and vascular development in the eye; circadian regulation of endocrine function; role of circadian clock in inflammation in retina
Robert Silverman, PhD (Cancer Biology)
OAS-RNase: principal mechanism of antiviral innate immunity by cleaving viral and cellular RNA, inhibiting virus replication and initiating apoptosis; cleaving of self-RNA triggering type I IFN response; evasion of IFN system by certain coronaviruses (MHV and MERS-CoV) and rotaviruses by encoding phosphodiesterases degrading 2-5A, preventing RNase L activation.
Jonathan Smith, PhD (Cardiovascular and Metabolic Sciences)
Genes altering atherosclerosis susceptibility in a mouse model; HDL metabolism, reverse cholesterol transport; genetics and functional genomics of atrial fibrillation; cell/molecular biology; biochemistry; genetics/genomics
George Stark, PhD (Cancer Biology)
Cell signaling mechanisms underlying innate immunity and cancer; insertional mutagenesis; responses to interferon; interferon synthesis in cancer; PD-L1 modulation of cellular response to IFN-1; novel therapies
Dennis Stuehr, PhD (Inflammation and Immunity)
Regulation of nitric oxide (NO) biosynthesis, impact of NO synthesis on cells and tissues; NO synthase chemistry, structure-function relationship, interaction with cellular proteins controlling activity; NO regulation of heme insertion into cellular proteins; protein nitration; protein nitration, its regulation and its control of protein function
Wai Hong Wilson Tang, MD (Cardiovascular and Metabolic Sciences)
Mechanistic determinants of inflammation and cellular processes in heart failure and cardiomyopathy; genetic, proteomic, and metabolomic links to pathogenic pathways in human heart diseases and heart failure; metabolites and proteins measured by mass spectroscopy techniques or immunoassays; enzyme expression in explanted human hearts
Bruce Trapp, PhD (Neurosciences)
Cellular/molecular biology of myelination; pathogenesis of neurological disability in multiple sclerosis
Oliver Wessely, PhD (Cardiovascular and Metabolic Sciences)
Kidney development and kidney diseases; molecular mechanisms governing the formation and maintenance of a functional kidney and pathophysiology in diseases; polycystic kidney disease; focal segmental glomerulosclerosis; renal cell carcinoma
Bin Zhang, PhD (Genomic Medicine)
Protein secretion dysregulation in disease development, cargo receptors impact on bleeding disorders; vesicle formation, COPII, and human disease; engineering gene therapies
Contact Info
Mailing Address
Cleveland State University
Dept. of Chemistry
2121 Euclid Ave., CHM
Cleveland, OH 44115-2214
Campus Location
Science & Research Center (SR)
2351 Euclid Avenue, Rm. 397
Contact Us
Phone: 216.687.2451
Fax: 216.687.9298
m.r.jones@csuohio.edu